Section 04: Legislation
Recent breakthroughs in PA legislation demonstrate the industry’s commitment to modernizing healthcare and protecting patients. Such legislation can make the PA submission process faster and easier for providers while preventing treatment delays for patients.
Learn About Federal & State Progress
Federal Developments
H.R. 6, the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act, was signed into law on October 24, 2018.22
Within H.R. 6 is a provision (Section 6062) stipulating utilization of ePA for drugs covered under Medicare Part D by January 1, 2021. This law requires the Centers for Medicare and Medicaid Services (CMS) to install regulations mandating Medicare Part D plans to accept PA requests submitted electronically and requires that the Secretary of Health and Human Services consult with standards development organizations, including NCPDP.
The opportunity to adopt the NCPDP SCRIPT Standard to expedite medication PA is an opportunity for the Secretary to ensure the Medicare Part D program is utilizing a prevalent standard used in other markets today. The standard named by the Secretary must be implemented.
Types of Legislation
Beyond progress at the federal level, many states are also adopting legislation around PA and ePA. Such laws range from use of a standardized form for submission to mandating the use of the NCPDP SCRIPT Standard for ePA.
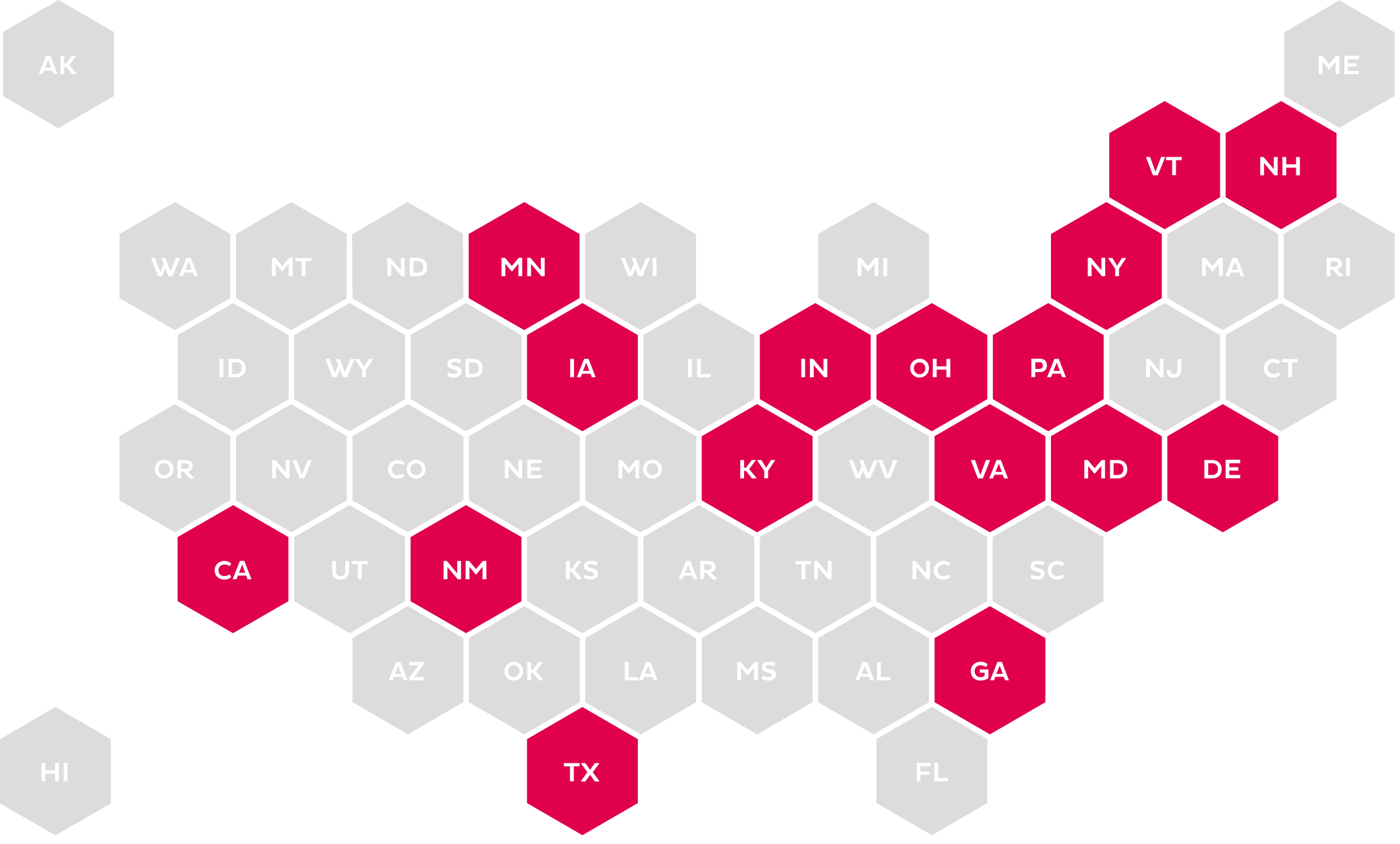
ePA (NCPDP STANDARD)
Calls for the use of an electronic method for submitting medication PA in compliance with the NCPDP SCRIPT Standard.
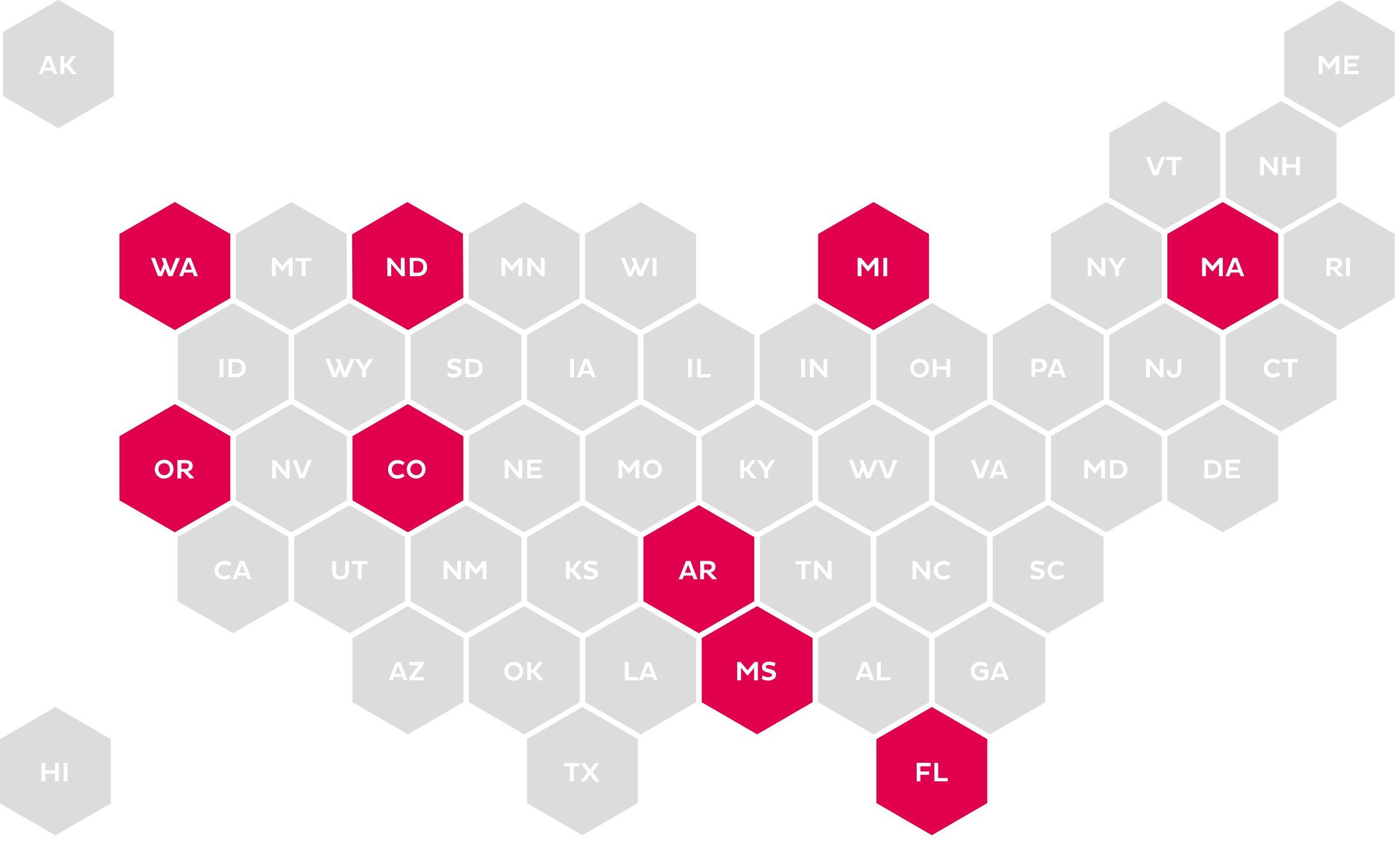
ePA (NO STANDARD)
Calls for the use of an electronic method for submitting medication PA, but names no standard.
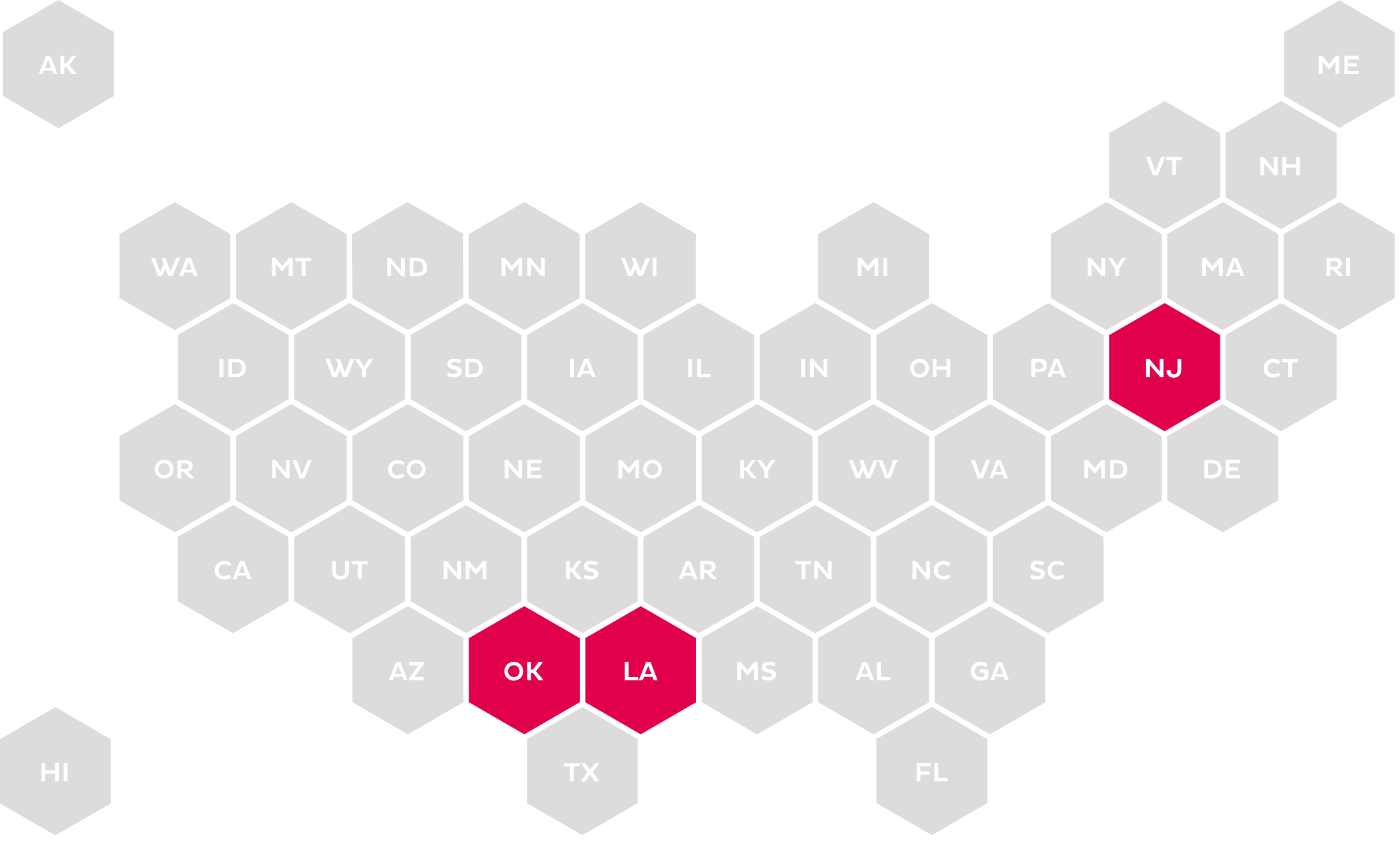
Standard Form
Calls for the use of a universal or standard form for medication PA approved by the state’s Department of Insurance.
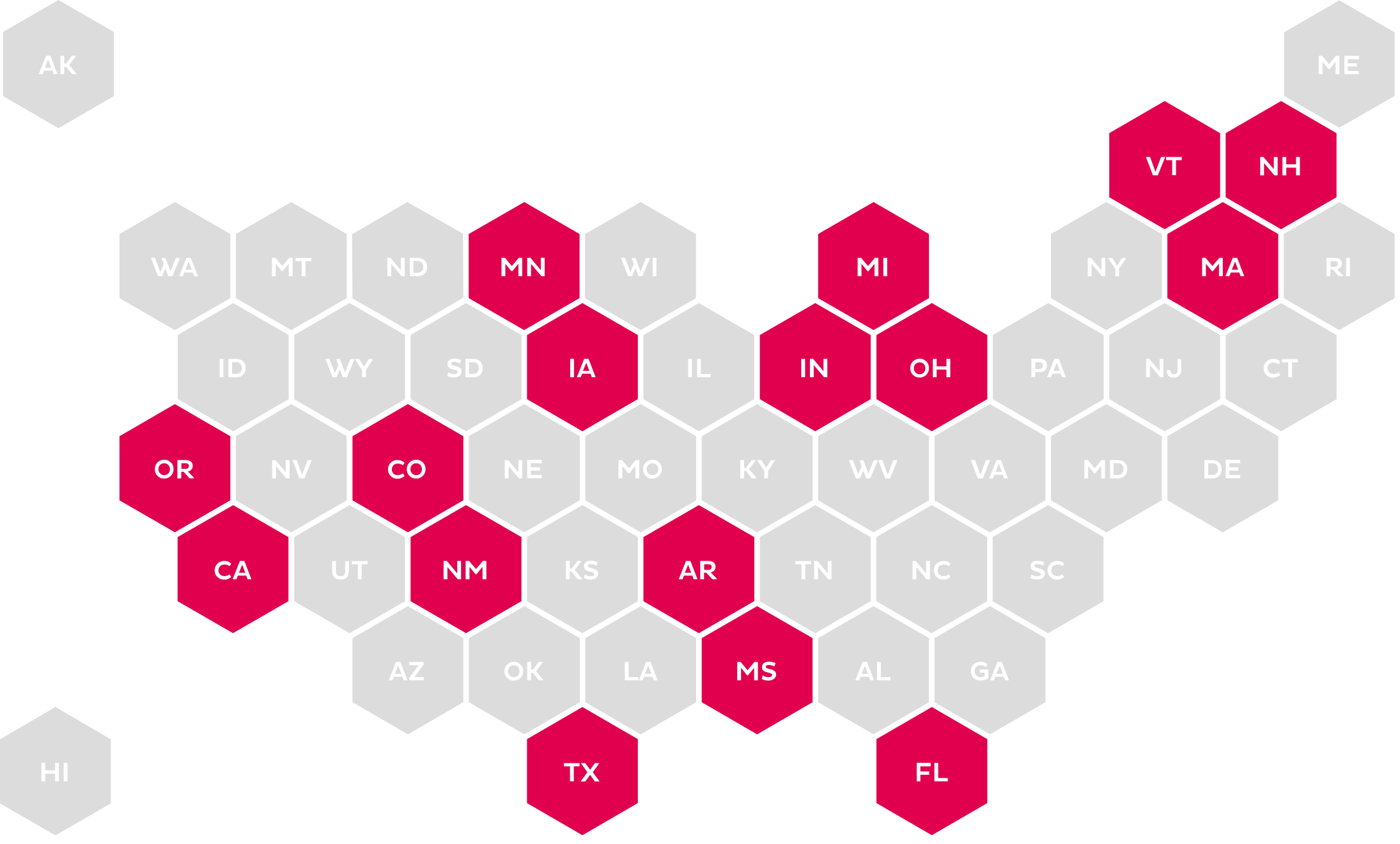
Standard Form & ePA
Calls for the use of a universal or standard form as well as the use of an electronic method for submitting medication PA.
Legislation By State
The increase in legislation around PA and ePA has become more prevalent at the state level. The mandates range from use of a standardized form for submission to mandating the use of the NCPDP SCRIPT Standard for ePA.23
Considerations for Standard Form
Legislation around PA often has a positive impact on the industry; however, it is important for new legislation to align with the most cutting-edge ePA technologies to make the most impact on streamlining PA.
While well-intentioned, laws requiring exclusive use of a standard form (despite pre-exisiting availability of ePA solutions) can inadvertently displace inefficiency, as the standard form cannot address all specific-use requirements that can arise during the PA process for various medications. As a result, providers, pharmacists and payers spend more time in back-and-forth phone conversations to resolve outstanding inquiries.23
Moving forward, it is critical for state-legislators to consider the availability of ePA solutions already active in their states and allow provisions for its use in standard form legislation to prevent unnecessary burden.
Medicaid and ePA
A study indicated specialty drugs account for nearly 33 percent of the total Medicaid drug spend, despite only 0.9 percent of the nearly 75 million Medicaid patients utilizing specialty medications.24, 25
Because non-adherence is a common problem among the Medicaid population, and an increasing number of patients are prescribed specialty medications,15 it’s vital that states latch on to an ePA solution.
A patient with limited resources and extenuating circumstances, such as lack of transportation, is more likely to be non-adherent. Non-adherence could lead to hospitalization, especially for patients on expensive specialty medications, which can ultimately create a costlier burden for states. An integrated ePA solution could lessen these issues for Medicaid patients and assist with medication readiness and adherence.
Key Takeaway
Across the country, advocacy for ePA has been rewarded as the federal government passed H.R. 6 requiring utilization of ePA for Medicare Part D drugs. States also continued to pass legislation supporting ePA; however, as legislation continues to develop, care must be taken to consider the practical, long-term effects on all stakeholders.